Abstract
Background: TP53 mutations are present in up to 20% of patients with myeloid malignancies and are frequently associated with a complex karyotype (CK) and poor responses to therapy. Even though overall response rates have improved significantly with less intensive induction modalities such as hypomethylating (HMA) agents alone or in combination with venetoclax, most patients succumb to their disease. AlloBMT remains the only curative option and even though the outcomes after alloBMT are disappointing, some patients achieve long-term survival. In the current study, we aimed to determine the clinical outcome of patients with TP53-MN and the impact of therapies including alloHCT on outcomes.
Methods: We performed a single-center retrospective review of adult patients with TP53-MN evaluated at Johns Hopkins between November 2015 and February 2020. The analysis of somatic mutations was performed using a 59-gene targeted next-generation sequencing (NGS) assay. Cytogenetic analysis was performed on bone marrow or peripheral blood using standard methods including G-banding by trypsin with Giemsa staining.
Results: We identified a total of 93 patients with TP53-MN. Disease subtypes included de novo AML (n = 22), AML arising from MDS/MPN (n = 15), MDS (n = 23), therapy-related myeloid neoplasm (n = 29), MDS/MPN overlap syndrome (n = 2) and MPN (n = 2). The median (Q1, Q3) age was 67 years (59 - 73 years). Cytogenetics was available for 89/93 (95.7%) patients. Of those, 73 (82.0%) patients had complex karyotype (CK). The median maximum TP53 variant allele frequency (VAF) per patient was 42.5%. Sixty-two (66.6%) patients had TP53 null phenotype: 14 (15.1%) patients had multiple TP53 mutations, 37 (41.6%) had deletion 17p and 11 (12.0%) had copy-neutral loss-of-heterozygosity (CnLOH).
Overall, 79/93 (85%) patients received therapy: 20 (25.3%) were treated with HMA alone, 30 (38.0%) with HMA+venetoclax, 10 (12.7%) with HMA+others, 15 (19.0%) with intensive chemotherapy, 2 (2.5%) with an immunomodulatory drug, 1 (1.3%) with ivosidenib and 1 (1.3%) with a JAK2 inhibitor. Of those, 29 (36.7%) patients received non-myeloablative alloBMT with post-transplant cyclophosphamide (10 (34.5%) bone marrow and 19 (65.5%) mobilized peripheral blood grafts (PBSCT)).
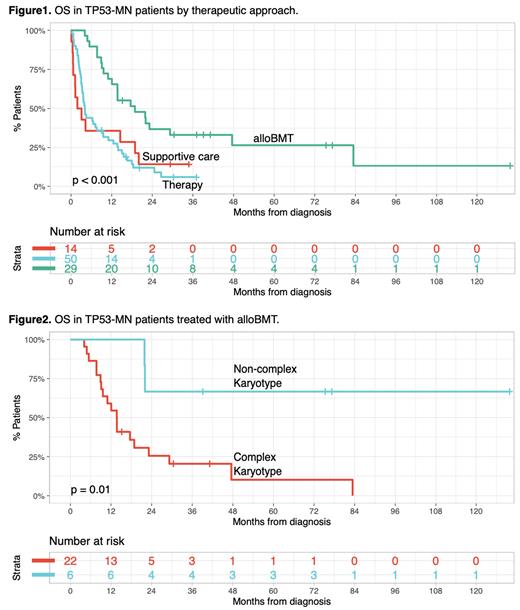
Median overall survival (OS) in patients receiving alloBMT, any therapy, or supportive care was 18.9, 4.1, and 2.5 months respectively (p < 0.001, Figure 1).
Among patients in the alloBMT group, there was a significant OS difference between TP53-MN CK vs. non-CK (median OS 13.7 months vs not reached, p = 0.01, Figure 2). Also, patients with TP53-MN CK demonstrated better OS when treated with alloBMT compared to any therapy or supportive care. However, most TP53-MN CK patients ultimately succumbed to their disease regardless of the therapeutic modality.
Therapeutic modalities prior to alloBMT did not appear to affect post-alloBMT outcomes but among patients who received PBSCT, OS was significantly better in patients receiving 400cGy vs 200 cGy total body irradiation (median OS 8.9 vs. 21 months, p=0.01).
Conclusions: TP53-MN appears to be a heterogeneous disease with a variable prognosis. While OS of patients with TP53 mutations and non-CK who underwent alloBMT resembled that of intermediate-risk AML, patients with TP53 null phenotype associated with CK had a worse outcome. Even though OS appears to be significantly improved with alloBMT, particularly patients receiving PBSCT+400cGy TBI, most patients eventually relapse. Novel therapeutic strategies are urgently needed to improve the outcomes of TP53 CK patients.
Disclosures
Jain:Care Dx, Bristol Myers Squibb, Incyte, Abbvie, CTI, and Kite: Other: Advisory Board participation; CTI Biopharma, SyneosHealth, Incyte: Research Funding. Ghiaur:Menarini Group: Consultancy, Research Funding; Syros Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Scripps: Consultancy, Research Funding. DeZern:CTI BioPharma: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Syntrix Pharmaceuticals: Research Funding; GERON: Other: DSMB. Gojo:Ono Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; Clearview: Membership on an entity's Board of Directors or advisory committees; Certara: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Immunogen: Membership on an entity's Board of Directors or advisory committees; Celgene: Other: Research support; Genentech: Other: Research support; Gilead: Membership on an entity's Board of Directors or advisory committees, Other: Research support; Amphivena: Other: Research Support; Merck: Other: Research support; Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Research Support; Johnson & Johnson: Membership on an entity's Board of Directors or advisory committees. Webster:Pfizer: Consultancy; Amgen: Consultancy. Levis:Astellas, and FujiFilm: Research Funding; AbbVie, Amgen, Astellas, Bristol Myers Squibb, Daiichi-Sankyo, FujiFilm, Jazz Pharmaceuticals, and Menarini: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal